
In an experiment, potassium chlorate decomposed according to the following chemical equation.
KClO3 → KCl + O2
(Molar mass of KClO3 = 122.5 g/mol; KCl = 74.55 g/mol; O2 = 31.998 g/mol)
If the mass of potassium chlorate was 240 grams, which of the following calculations can be used to determine the mass of oxygen gas formed?
(240 × 2 × 31.998) ÷ (122.5 × 3) grams
(240 × 3 × 31.998) ÷ (122.5 × 2) grams
(240 × 2 × 122.5) ÷ (31.998 × 3) grams
(240 × 3 × 122.5) ÷ (31.998 × 2) grams

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:10
How do forces between particles in gases compare to forces in the other states of matter? o a. the forces in gases are stronger than forces in solids but weaker than forces in liquids. o b. the forces in gases are weaker than forces in solids but stronger than forces in liquids. o c. the forces in gases are weaker than forces in solids and liquids. o d. the forces in gases are stronger than forces in solids and liquids. submit
Answers: 1

Chemistry, 22.06.2019 01:00
Water is important for the of cells. a: size, shape, and temperature b: temperature, color, and odor c: color, odor, and size d: shape, temperature, and color
Answers: 2

Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1

Chemistry, 23.06.2019 01:00
If a sample of radioactive isotopes takes 600 minutes to decay from 400 grams to 50 grams, what is the half-life of the isotope?
Answers: 1
You know the right answer?
In an experiment, potassium chlorate decomposed according to the following chemical equation.
KClO3...
Questions

Mathematics, 14.07.2020 14:01



Mathematics, 14.07.2020 14:01

Mathematics, 14.07.2020 14:01

Mathematics, 14.07.2020 14:01


English, 14.07.2020 14:01





Chemistry, 14.07.2020 14:01

Chemistry, 14.07.2020 14:01




Mathematics, 14.07.2020 14:01


Mathematics, 14.07.2020 14:01

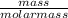
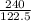
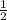 x 31.998
x 31.998

