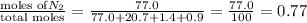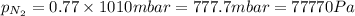Chemistry, 18.01.2021 21:10 jones501324
1. Consider the following properties of the atmosphere near the coast in Southern California: Average surface pressure: 1010 mbar Average temperature: 295 K Atmospheric gas composition (by volume): Nitrogen (N2) 77.0%, Oxygen (O2) 20.7%, Water (H2O) 1.4%, Argon (Ar), 0.9%. a. What is the average partial pressure of N2 in the atmosphere, pA, in units of Pa

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1


Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1
You know the right answer?
1. Consider the following properties of the atmosphere near the coast in Southern California: Averag...
Questions

Spanish, 20.11.2020 22:30




English, 20.11.2020 22:30

Mathematics, 20.11.2020 22:30

Mathematics, 20.11.2020 22:30


Computers and Technology, 20.11.2020 22:30

Mathematics, 20.11.2020 22:30

Biology, 20.11.2020 22:30



Social Studies, 20.11.2020 22:30



Advanced Placement (AP), 20.11.2020 22:30


Spanish, 20.11.2020 22:30

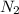 in the atmosphere is 77770 Pa.
in the atmosphere is 77770 Pa.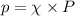
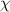 = mole fraction
= mole fraction 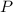 = total pressure
= total pressure