
Chemistry, 14.01.2021 18:10 smallblueberry
i) Mass of CO2 formed = 2.241 g Molar mass of CO2 = Atomic mass of C + 2 (Atomic mass of O) = 12 + 2 (16) g/mol = 44 g/mol Thus, moles of carbon dioxide formed

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 23.06.2019 03:30
In general metals get as you move from left to right across the periodic table.
Answers: 1
You know the right answer?
i) Mass of CO2 formed = 2.241 g Molar mass of CO2 = Atomic mass of C + 2 (Atomic mass of O) = 12 + 2...
Questions


Mathematics, 23.06.2019 13:30

Social Studies, 23.06.2019 13:30


Mathematics, 23.06.2019 13:30

Mathematics, 23.06.2019 13:30

Mathematics, 23.06.2019 13:30

Mathematics, 23.06.2019 13:30






Chemistry, 23.06.2019 13:30



Mathematics, 23.06.2019 13:30



Mathematics, 23.06.2019 13:30

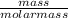
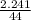 = 0.05mole
= 0.05mole

