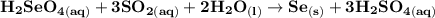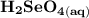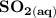Chemistry, 14.01.2021 16:40 angelreji386
Write a balanced equation describing the reduction of H2SeO4 by SO2 to produce selenium. (Use the lowest possible coefficients. Include states-of-matter under SATP conditions in your answer.)

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3

Chemistry, 23.06.2019 01:00
Imagine if during the cathode ray experiment, the size of the particles of the ray was the same as the size of the atom forming the cathode. which other model or scientific observation would have also been supported? 1. this would support dalton's postulates that proposed the atoms are indivisible because no small particles are involved. 2. this would support bohr's prediction about electrons moving in orbits having specific energy. 3. this would support bohr's prediction about electrons being randomly scattered around the nucleus in the atom. 4. this would support dalton's postulates that proposed that atoms combine in fixed whole number ratios to form compounds.
Answers: 1

Chemistry, 23.06.2019 07:30
How many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride
Answers: 1
You know the right answer?
Write a balanced equation describing the reduction of H2SeO4 by SO2 to produce selenium. (Use the lo...
Questions

English, 08.12.2020 06:50


Biology, 08.12.2020 06:50









Biology, 08.12.2020 06:50




English, 08.12.2020 06:50

History, 08.12.2020 06:50

Chemistry, 08.12.2020 06:50