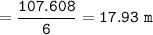Chemistry, 10.01.2021 16:40 emmadivaburnsox7ae9
Winter is coming and it's time to make sure you have the right amount of antifreeze
in your car. The best mixture of antifreeze and water is 50% antifreeze with 50%
water. The cooling system in your car has a mixture of 6.00 L water with 6.00 L of
ethylene glycol (antifreeze).
What is the molality of that solution?
The chemical formula for ethylene glycol is C2H602 and its density is 1.1132 g/cm3. (
Hint- use the density to find the mass of 6.0 L of C2H6O2, and remember that 1.0cm3
= 1.0 mL and that 1.0 L of water = 1.0 kg)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:10
Nitrogen (n), phosphorus (p), and potassium (k) are the main nutrients in plant fertilizers. according to an industry convention, the numbers on the label refer to the mass percents of n, p2o5, and k2o, in that order. calculate the n: p: k ratio of a 30: 10: 10 fertilizer in terms of moles of each element, and express it as x: y: 1.0.
Answers: 1

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 23.06.2019 00:20
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1

Chemistry, 23.06.2019 15:00
Does the formation of all chemical bonds is based on sharing of electrons?
Answers: 1
You know the right answer?
Winter is coming and it's time to make sure you have the right amount of antifreeze
in your car. Th...
Questions





Social Studies, 08.10.2019 19:40




Business, 08.10.2019 19:40


English, 08.10.2019 19:40



Mathematics, 08.10.2019 19:40


Biology, 08.10.2019 19:40

History, 08.10.2019 19:40

Health, 08.10.2019 19:40


Advanced Placement (AP), 08.10.2019 19:40