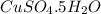What is the formula for the compound copper (II) sulfate pentahydrateA. C2S3•6H2O
C
2
S...

Chemistry, 09.01.2021 22:10 kaidencearley
What is the formula for the compound copper (II) sulfate pentahydrateA. C2S3•6H2O
C
2
S
3
•
6
H
2
O
B. CuSO4•6H2O
C
u
S
O
4
•
6
H
2
O
C. C2S3•5H2O
C
2
S
3
•
5
H
2
O
D. CuSO4•5H2O

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:20
Which of the following statements is false regarding aromaticity? a. the compound must be cyclic b. the compound must be fully conjugated c. the compound must be planar d.the number of electrons in the pi system must satisfy the hückel 4n+2 rule e. the compound must have a neutral charge
Answers: 2

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1

Chemistry, 23.06.2019 01:50
Drag the tiles to the correct locations. each tile can be used more than once, but not all tiles will be used. one or more locations will remain empty. nitrosyl fluoride has the chemical formula nof nitrogen has five valence electrons, oxygen has six, and fluorine has seven. complete the lewis structure for this covalent compound. f n = = = . : : 0 : reset next um. all rights reserved us 2
Answers: 2
You know the right answer?
Questions








Computers and Technology, 22.07.2021 19:10




Biology, 22.07.2021 19:10


Chemistry, 22.07.2021 19:10





History, 22.07.2021 19:10