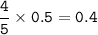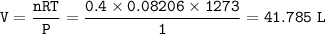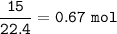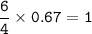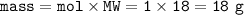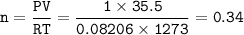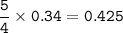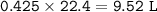Catalyst
A reaction between NH, and O, is the first step in the
preparation of nitric acid (H...

Catalyst
A reaction between NH, and O, is the first step in the
preparation of nitric acid (HNO3) on a commercial scale.
The products are produced at 1000°C (1273 K) and at at-
mospheric pressure.
4 NH; (g) + 5 O2 (g) → 4 NO (g) + 6 H2O (1)
a. What volume of NO is produced in the reaction vessel
by the reaction of 0.500 mol O2?
b. What mass of H2O is produced by the reaction of 15.0 L
of NH3?
c. How many liters of O, must react to produce 35.5 L of
NO?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:00
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1

Chemistry, 23.06.2019 00:00
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3
You know the right answer?
Questions

Mathematics, 04.08.2019 08:00

Social Studies, 04.08.2019 08:00



English, 04.08.2019 08:00


Arts, 04.08.2019 08:00





History, 04.08.2019 08:00

Health, 04.08.2019 08:00

Biology, 04.08.2019 08:00

History, 04.08.2019 08:00

Social Studies, 04.08.2019 08:00

Mathematics, 04.08.2019 08:00

Mathematics, 04.08.2019 08:00