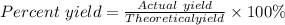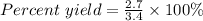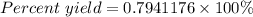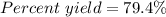Chemistry, 01.01.2021 04:50 timothycarter342
Sulfuric acid, H 2 S O 4 , is an important industrial chemical, typically synthesized in a multi-step process. What is the percent yield if a batch of H 2 SO 4 has a theoretical yield of 3.4 kg, and 2.7 kg are obtained at the end of the process

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:00
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 23.06.2019 01:30
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2
You know the right answer?
Sulfuric acid, H 2 S O 4 , is an important industrial chemical, typically synthesized in a multi-ste...
Questions



Medicine, 26.02.2021 01:30


Health, 26.02.2021 01:30




Mathematics, 26.02.2021 01:30

Mathematics, 26.02.2021 01:30


Biology, 26.02.2021 01:30




Physics, 26.02.2021 01:30

History, 26.02.2021 01:30