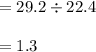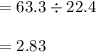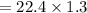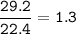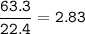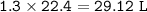Methane gas combusts according to the following
chemical equation:
CH4 (8) + 202(g) → CO2(g)...

Methane gas combusts according to the following
chemical equation:
CH4 (8) + 202(g) → CO2(g) + 2H2O(l)
29.2 L of methane gas is combusted with 63.3 L of
oxygen gas at STP. What volume of carbon dioxide
is produced in the reaction?
___L CO2
Your answer should be rounded to three significant figures. Do
not include units in your answer.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 22.06.2019 18:00
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1
You know the right answer?
Questions