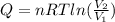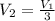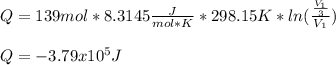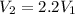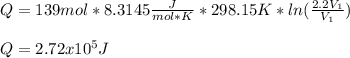An ideal gas resides in a closed cylinder (diameter is 0.5 ft) with a frictionless piston. The initial conditions are 139 mol of the ideal gas at 25°C. The piston is compressed isothermally to one third of the initial volume. The heat capacity C_v of an ideal gas is 1.5 middot R.
Required:
a. What is the heat interaction (kJ) for this process?
b. The piston now expands isothermally to 120% of the same initial volume. Will the heat interaction increase, decrease, or stay the same?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

You know the right answer?
An ideal gas resides in a closed cylinder (diameter is 0.5 ft) with a frictionless piston. The initi...
Questions



Mathematics, 02.04.2021 02:50

Spanish, 02.04.2021 02:50

English, 02.04.2021 02:50

Chemistry, 02.04.2021 02:50






Mathematics, 02.04.2021 02:50


Biology, 02.04.2021 02:50


Physics, 02.04.2021 02:50