
Chemistry, 16.12.2020 14:00 ashvinmsingh
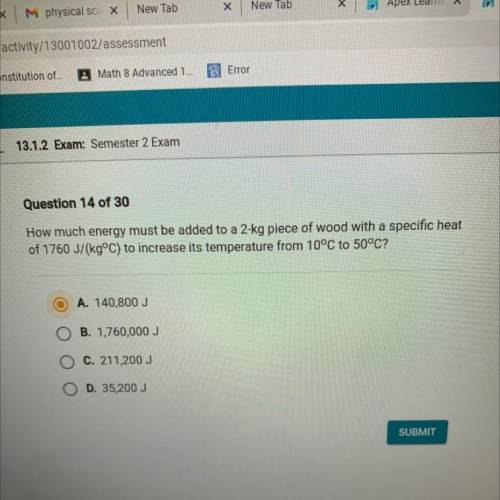
How much energy must be added to a 2-kg piece of wood with a specific heat
of 1760 J/(kg°C) to increase its temperature from 10°C to 50°C?
A. 140,800 J
B. 1,760,000 J
C. 211,200 J
D. 35,200 J

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:10
Atank contains 240 liters of fluid in which 10 grams of salt is dissolved. brine containing 1 gram of salt per liter is then pumped into the tank at a rate of 6 l/min; the well-mixed solution is pumped out at the same rate. find the number a(t) of grams of salt in the tank at time t.
Answers: 3

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1

Chemistry, 22.06.2019 23:30
The sum of the oxidation numbers in a neutral compound is always
Answers: 2

Chemistry, 23.06.2019 00:00
This statement about matter and its behavior is best classified as a
Answers: 1
You know the right answer?
How much energy must be added to a 2-kg piece of wood with a specific heat
of 1760 J/(kg°C) to incr...
Questions

Mathematics, 20.05.2021 02:50

Mathematics, 20.05.2021 02:50

Chemistry, 20.05.2021 02:50



History, 20.05.2021 02:50



Biology, 20.05.2021 02:50


Mathematics, 20.05.2021 02:50




History, 20.05.2021 02:50

Mathematics, 20.05.2021 02:50

History, 20.05.2021 02:50

Mathematics, 20.05.2021 02:50

Mathematics, 20.05.2021 03:00

History, 20.05.2021 03:00



