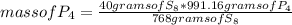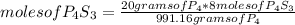Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 09:00
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

You know the right answer?
8P4 + 3S8 -> 8P4S3
The molar masses of the substances are as follows: P4 = 123.895g/mol, S8 = 25...
Questions

Mathematics, 23.09.2020 19:01











Biology, 23.09.2020 19:01




Health, 23.09.2020 19:01

History, 23.09.2020 19:01



Chemistry, 23.09.2020 19:01