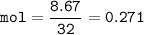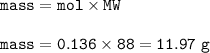Chemistry, 12.12.2020 23:50 lclaudettecarte4720
Fe(s) + S(s) → FeS(s)
In one experiment, 7.62 g of Fe are allowed to react with 8.67 g of S.
What is the limiting reagent, and what is the reactant in excess?
Calculate the mass of FeS formed.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Write the chemical symbols for three different atoms or atomic cations with 27 electrons. asap!
Answers: 2

Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 23.06.2019 09:00
Agust of wind blowing east pushes against a ball. when will the wind do work on the ball? when the ball moves to the east when the ball moves to the north when the ball stays in one place when the ball moves north or south
Answers: 1

Chemistry, 23.06.2019 09:30
What is the force of an object when it landed(sitting in the ground)
Answers: 2
You know the right answer?
Fe(s) + S(s) → FeS(s)
In one experiment, 7.62 g of Fe are allowed to react with 8.67 g of S.
...
...
Questions





Biology, 11.07.2019 11:00

Mathematics, 11.07.2019 11:00

Biology, 11.07.2019 11:00

Chemistry, 11.07.2019 11:00


Mathematics, 11.07.2019 11:00

Biology, 11.07.2019 11:00



Biology, 11.07.2019 11:00


Mathematics, 11.07.2019 11:00

Biology, 11.07.2019 11:00