
Chemistry, 12.12.2020 16:00 adelawilliams60
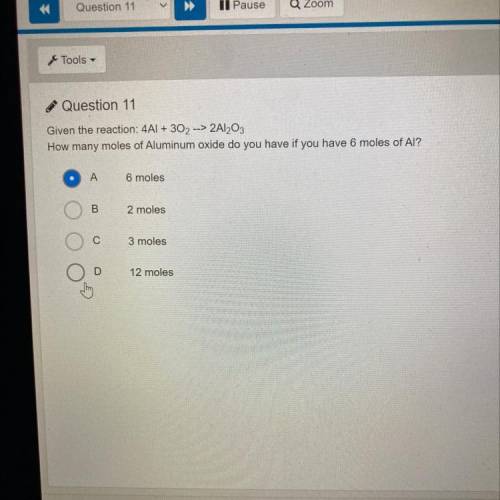
Given the reaction: 4Al + 302 --> 2AI2O3
How many moles of Aluminum oxide do you have if you have 6 moles of AI?
A
6: moles
B: 2 moles
C: 3 moles
D: 12 moles

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
After cloud droplets form, what must happen to them for precipitation to occur?
Answers: 1

Chemistry, 22.06.2019 00:00
How did planetesmals form planets? a. they broke apart into smaller chunks.b. they collided and stuck together.c. they cooled and pulled ice together.d. they began to rotate.
Answers: 1

Chemistry, 22.06.2019 10:30
Balance and in which category does it fit in? single or double displacement or synthesis or decomposition? (a) k2 o → k + o2 (b) na + i2 → nai (c) cu(no3 )2 + naoh → cu(oh)2 + nano3 (d) kclo3 → kcl + o2 (e) ca(no3 )2 + hbr → cabr2 + hno3 (f) sn(oh)2 → sno + h2 o (g) p4 + n2 o → p4 o6 + n2 (h) fe + al2 (so4 )3 → feso4 + al (i) alcl3 + na2 co3 → al2 (co3 )3 + nacl (j) c3 h6 + o2 → co2 + h2 o
Answers: 1

Chemistry, 22.06.2019 14:30
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4.0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3
You know the right answer?
Given the reaction: 4Al + 302 --> 2AI2O3
How many moles of Aluminum oxide do you have if you hav...
Questions

English, 10.11.2020 06:30


Physics, 10.11.2020 06:30

Health, 10.11.2020 06:30

Mathematics, 10.11.2020 06:30

Mathematics, 10.11.2020 06:30

Spanish, 10.11.2020 06:30



Health, 10.11.2020 06:30

Social Studies, 10.11.2020 06:30

Engineering, 10.11.2020 06:30

Mathematics, 10.11.2020 06:30

English, 10.11.2020 06:30





History, 10.11.2020 06:30

Biology, 10.11.2020 06:30



