
Chemistry, 12.12.2020 15:50 nathancikra
5.00 ml of an H2SO4 solution with unknown molarity was diluted with 25.00 ml. This was tirated with 15.00ml of .1000 M NaOH to reach the equivalence point. What is the molarity of the original unknown H2SO4 solution? Also, what would the balanced chemical equation for this reaction be?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1

Chemistry, 22.06.2019 16:00
1. an experiment in your science class lists the materials needed for the lab. it is your job, as a lab partner, to measure out 25 ml of distilled water and 2.5 grams of magnesium. what lab measuring tools would you choose to measure each substance and how would you use each tool to get the correct amounts? be sure to describe the process you would follow step-by-step. (5 points) 2.which of the following is an si base unit for measuring mass? (2 points) ampere gram meter pound 3.which of the following is an si base unit for time? (2 points) decades hours minutes seconds 4.which of the following tools should a scientist use to measure an object in milligrams? (2 points) graduated cylinder pan balance tape measure thermometer 4.which of the following tools should a scientist use to measure an object in milligrams? (2 points) graduated cylinder pan balance tape measure thermometer. 5.a pencil beside a metric ruler. the ruler is scaled from 1 centimeter to 10 centimeters, with markings for millimeters between each number. one end of the pencil is beside the 0 on the ruler, and the pencil point is beside the 5. which of the following measurements is accurate but not precise? (2 points) 5 mm 5 cm 50 mm 50 cm 6. which of the following prefixes represents the largest value? (2 points) giga hector kilo milli 7. which of the following types of graphs is best for plotting the percentages of a whole value in a data set? (2 points) bar graph circle graph histogram line graph
Answers: 1

Chemistry, 22.06.2019 21:40
Tooth enamel consists mainly of the mineral calcium hydroxyapatite, ca_10(po_4)_6(oh)_2. trace elements in teeth of archaeological specimens provide anthropologist with clues about diet and diseases of ancient people. students at hamline university measured strontium in enamel from extracted wisdom teeth by atomic absorption spectroscopy. solutions with a constant total volume of 10.0 ml contained 0.726 mg of dissolved tooth enamel plus variable concentrations of added sr. added sr find the concentration of sr in the 10 ml sample solution in parts per billion = ng/ml. find the concentration of sr in tooth enamel in parts per million = mu g/g.
Answers: 2

Chemistry, 23.06.2019 03:30
The molar mass of nickel(ni) is 58.7 g/mol. how many moles are in an 88 gram sample of nickel?
Answers: 1
You know the right answer?
5.00 ml of an H2SO4 solution with unknown molarity was diluted with 25.00 ml. This was tirated with...
Questions

Mathematics, 24.05.2021 21:20



Biology, 24.05.2021 21:20

English, 24.05.2021 21:20

Mathematics, 24.05.2021 21:20

English, 24.05.2021 21:20

Chemistry, 24.05.2021 21:20


Chemistry, 24.05.2021 21:20

Mathematics, 24.05.2021 21:20

Mathematics, 24.05.2021 21:20



Mathematics, 24.05.2021 21:20

Mathematics, 24.05.2021 21:20

Chemistry, 24.05.2021 21:20

History, 24.05.2021 21:20

Chemistry, 24.05.2021 21:20

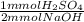 = 0.75 mmol H₂SO₄
= 0.75 mmol H₂SO₄

