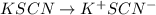Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 06:20
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3

You know the right answer?
If 45.0 mL of a 0.0500 M HNO3, 10.0 mL of a 0.0500 M KSCN, and 30.0 mL of a 0.0500 M Fe(NO3)3 are co...
Questions

English, 20.11.2019 23:31

Mathematics, 20.11.2019 23:31





Chemistry, 20.11.2019 23:31

Mathematics, 20.11.2019 23:31


Arts, 20.11.2019 23:31


Biology, 20.11.2019 23:31

Social Studies, 20.11.2019 23:31


Mathematics, 20.11.2019 23:31





History, 20.11.2019 23:31