
Chemistry, 08.12.2020 21:20 aylengarcia090
PLEASE HELP THEY ARE EASY QUESTIONS THERE ARE JUST A LOT Please answer these. The tables needed for question 7 are in the picture. I got rid of some of the questions that you wouldn’t be able to answer without doing the lesson
Question 1: Electron Notation Example (2 points)
a. Give the electron configuration of vanadium (V), atomic number 23. (0.5 points)
b. Give the noble gas configuration of vanadium (V), atomic number 23. (0.5 points)
c. List the energy levels for the orbital configuration of vanadium (V), atomic number 23. (1 point)
Question 3: Trends on the Periodic Table (2 points)
a. How does the atomic radius change going down and across the periodic table? (0.5 points)
b. How does first ionization energy change going down and across the periodic table? (0.5 points)
c. How does electronegativity change going down and across the periodic table? (0.5 points)
d. How does the radius of a positive and negative ion compare to a neutral atom? (0.5 points)
Question 4: Chemical Bonds (1 point)
Match each chemical bond to its correct description. (1 point)
A. Ionic bond ___ Sharing of electrons
B. Covalent bond ___ Freely moving electrons
C. Metallic bond ___ Transfer of electrons
Question 5: Intermolecular Forces (3 points)
a. Describe the dipole-dipole force. (1 point)
b. Describe hydrogen bonding. (1 point)
c. Describe the Van der Waals forces. (1 point)
Question 6: Intermolecular Forces and You (2 points)
Imagine you need to take a medicine that the doctor has prescribed for you. Explain why scientists who developed that medicine would need to know whether or not the compound in that medicine is polar. How might a polar medicine behave differently within your body than a nonpolar medicine would? Answer in 1 to 2 paragraphs.
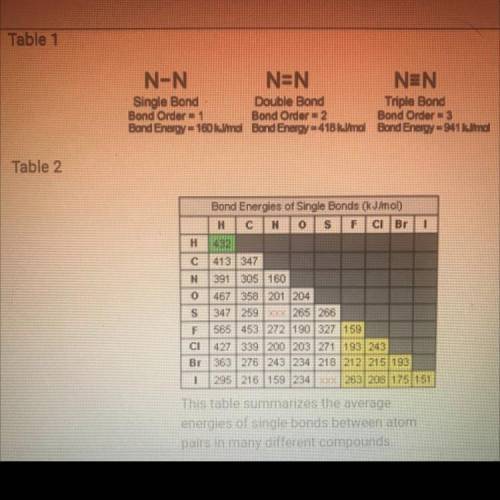
Question 7: Energy in Bonds (9 points)
Use these tables for reference for all parts of this question.
This table summarizes the average energies of single bonds between atom pairs in many different compounds.
a. According to Table 2, which is the strongest bond? Which is the weakest bond? Based on what you know about the atomic radii and electronegativity of the elements involved in the bonds, why do you think these two have the most extreme bond-energy values? (3 points)
b. How are the bond energies of each bond listed in Table 2 determined? (1 point)
c. Why do you think there aren't bond energy values given in Table 2 for N–S and S–I? (1 point)
d. Based on Tables 1 and 2, how would you describe the trend in bond strength of single, double, and triple bonds? (1 point)
e. Based on Table 2, how would you describe the trend in the strength of bonds formed by the elements carbon, nitrogen, and oxygen? Would you describe this trend as a periodic trend? Why or why not? (3 points)
Question 8: Causes of Molecular Shape (3 points)
a. What is the VSEPR theory? (1 point)
b. How does electron repulsion determine molecular shape? (1 point)
c. How do lone electron pairs affect molecular shape? (1 point)
Question 10: Lewis Structure (3 points)
a. Draw the Lewis structure for the Se and 2 H atoms. (1 point)
b. Draw the Lewis structure for the SeH2 molecule. (1 point)
c. What shape would SeH2 have? Draw the molecule. (1 point)
Question 11: Ionic and Covalent Compounds (5 points)
Identify each of the following as a covalent compound or ionic compound. Then provide either the formula for compounds identified by name or the name for those identified by formula. (1 point each)
a. Li2O:
b. Dinitrogen trioxide:
c. PCl3:
d. Manganese(III) oxide:
e. Calcium bromide:

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2


Chemistry, 22.06.2019 19:50
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2
You know the right answer?
PLEASE HELP THEY ARE EASY QUESTIONS THERE ARE JUST A LOT Please answer these. The tables needed for...
Questions




Computers and Technology, 16.12.2021 17:50

Computers and Technology, 16.12.2021 17:50




English, 16.12.2021 17:50

Mathematics, 16.12.2021 17:50

Mathematics, 16.12.2021 17:50


Physics, 16.12.2021 17:50

Mathematics, 16.12.2021 17:50




English, 16.12.2021 17:50

Social Studies, 16.12.2021 17:50




