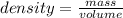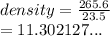Chemistry, 07.12.2020 17:30 Alijahvalles7443
The volume of water in a graduated cylinder was increased from 52.0 mL to 75.5 mL when a piece of irregular metal was placed in the water. If the piece of metal has a mass of 265.6 g, what is the density of the metal?

Answers: 3
Another question on Chemistry

Chemistry, 23.06.2019 14:30
Recognizing the properties of water water has a "bent" geometry. which explanation does not explain why? o water's oxygen has unbonded electron pairs that repel each other. water can form hydrogen bonds. electrons are evenly distributed in the water molecule. do ne
Answers: 3

Chemistry, 23.06.2019 19:00
What is the final temperature after 840 joules is absorbed by 10.0g of water at 25.0 c?
Answers: 1

Chemistry, 23.06.2019 22:40
Carbons 1 and 4 of 1,3−cyclopentadiene are equivalent and give the same carbocation on protonation. likewise, carbons 2 and 3 are equivalent. write the structure of the carbocation formed by protonation of c−2 or c−3 to verify that it is not allylic and therefore not as stable as the one formed by protonation of c−1 or c−4.
Answers: 1

Chemistry, 24.06.2019 03:00
In the industrial "chlor-alkali" process, pure chlorine and sodium hydroxide are produced by electrolyzing brine, essentially an aqueous solution of sodium chloride. suppose a current of is passed through an aqueous solution of for seconds. calculate the mass of pure chlorine produced. be sure your answer has a unit symbol and the correct number of significant digits.
Answers: 1
You know the right answer?
The volume of water in a graduated cylinder was increased from 52.0 mL to 75.5 mL when a piece of ir...
Questions

Mathematics, 18.11.2020 01:50



Physics, 18.11.2020 01:50



Mathematics, 18.11.2020 01:50

Mathematics, 18.11.2020 01:50

Health, 18.11.2020 01:50

Arts, 18.11.2020 01:50



Biology, 18.11.2020 01:50

Mathematics, 18.11.2020 01:50

Computers and Technology, 18.11.2020 01:50

Business, 18.11.2020 01:50




History, 18.11.2020 01:50