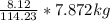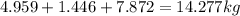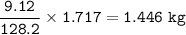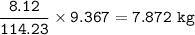Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2

Chemistry, 22.06.2019 22:30
What must be in balance for temperatures to remain constant?
Answers: 1
You know the right answer?
A mixture of hydrocarbons contains 34.8% heptane, C7H16, 10.1% nonane, C9H20, and 55.1% octane, C8H1...
Questions

Chemistry, 28.01.2020 23:06

History, 28.01.2020 23:06


English, 28.01.2020 23:06




English, 28.01.2020 23:06


Mathematics, 28.01.2020 23:06


History, 28.01.2020 23:06

Mathematics, 28.01.2020 23:06





Mathematics, 28.01.2020 23:06

Social Studies, 28.01.2020 23:06

Mathematics, 28.01.2020 23:06

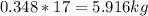 Mass C in Heptane (MW=100.21 g/mol) :
Mass C in Heptane (MW=100.21 g/mol) :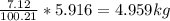
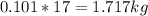 Mass C in Nonane (MW=128.2 g/mol) :
Mass C in Nonane (MW=128.2 g/mol) :
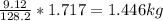
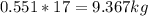 Mass C in Otane (MW=114.23 g/mol) :
Mass C in Otane (MW=114.23 g/mol) :