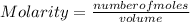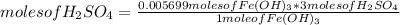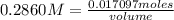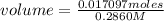Chemistry, 04.12.2020 17:00 kristinaholahan
How many mL of 0.2860 M sulfuric acid are required to react with 58.42 mL of 0.09756 M iron(III) hydroxide

Answers: 1
Another question on Chemistry


Chemistry, 23.06.2019 01:00
If a sample of radioactive isotopes takes 600 minutes to decay from 400 grams to 50 grams, what is the half-life of the isotope?
Answers: 1

Chemistry, 23.06.2019 09:30
How many moles of na2s2o3 are needed to react with 0.12mol of cl2? show work.
Answers: 1

Chemistry, 23.06.2019 15:30
In a modern periodic table, there are seven periods. a period is any horizontal row of the periodic table, and the elements in a period have consecutive atomic numbers. a group is any vertical column in the periodic table, and there are 18 such groups. groups 3–12, also known as the “b” group elements, are called transition metals. groups 1–2 and 13–18, also known as the “a” group elements, are sometimes called the main groups. metals are characterized by malleability, ductility, conductivity, and a tendency to lose electrons. main group metals are found in groups 1 and 2. nonmetallic elements fall on the right-hand side of the periodic table, that is, groups 13–18. nonmetals have the tendency to gain electrons and are generally brittle. they can be solids, liquids, or gasses at room temperature. now, label the areas of the modern periodic table using the above information. drag the appropriate labels to their respective targets.
Answers: 1
You know the right answer?
How many mL of 0.2860 M sulfuric acid are required to react with 58.42 mL of 0.09756 M iron(III) hyd...
Questions

Mathematics, 29.06.2019 06:30

English, 29.06.2019 06:30

Arts, 29.06.2019 06:30

Physics, 29.06.2019 06:30

Mathematics, 29.06.2019 06:30

Mathematics, 29.06.2019 06:30

History, 29.06.2019 06:30


Mathematics, 29.06.2019 06:30


History, 29.06.2019 06:30


Mathematics, 29.06.2019 06:30





Mathematics, 29.06.2019 06:30


History, 29.06.2019 06:30