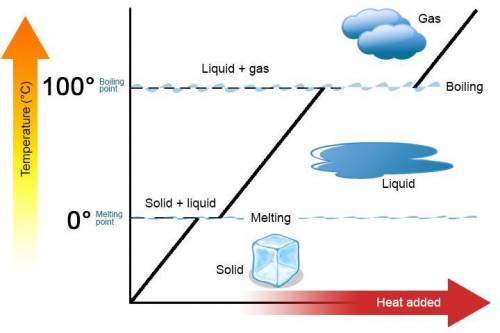
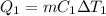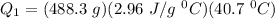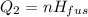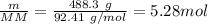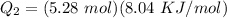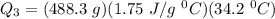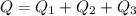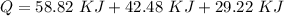A 488.3 gram sample of an unknown substance (MM = 92.41 g/mol) is heated from -23.1 °C to 51.8 °C. (heat capacity of solid = 2.96 J/g・°C; heat capacity of liquid = 1.75 J/g・°C; ∆Hfus = 8.04 kJ/mol; Tfinal = 17.6 °C)
a) How much energy (in kJ) is absorbed/released to heat the solid?
b)How much energy (in kJ) is absorbed/released to melt the solid?
c)How much energy (in kJ) is absorbed/released to heat the liquid?
d) What is the total amount of energy that must be absorbed/released for the entire process?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Your friend offers to show you an intrusive igneous rock. which of the following would you expect to see?
Answers: 1

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2

Chemistry, 22.06.2019 21:00
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1

Chemistry, 23.06.2019 04:31
Pls i will do pls imma diewhat forms white light? (4 points)a. combination of all wavelengths of ultraviolet light b. combination of all wavelengths of visible lightc. absorption of electromagnetic waves d. absorption of infrared rays
Answers: 2
You know the right answer?
A 488.3 gram sample of an unknown substance (MM = 92.41 g/mol) is heated from -23.1 °C to 51.8 °C. (...
Questions





Mathematics, 11.09.2019 03:30

History, 11.09.2019 03:30


Mathematics, 11.09.2019 03:30

History, 11.09.2019 03:30


English, 11.09.2019 03:30

Spanish, 11.09.2019 03:30

History, 11.09.2019 03:30


History, 11.09.2019 03:30

History, 11.09.2019 03:30

Mathematics, 11.09.2019 03:30



History, 11.09.2019 03:30