
Chemistry, 03.12.2020 18:10 fernandaElizondo
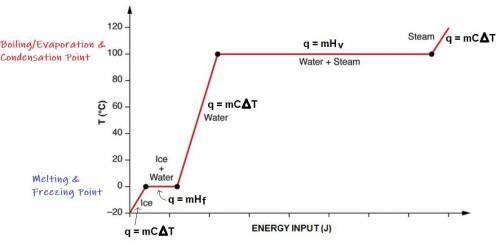
A 200 g sample of water at 60.0 degrees Celsius is heated to water vapor at 140.0 degrees Celsius. Expected Answer = 501,440 J Before trying to solve this problem, explain:
what is happening to the water from 60.0 degrees Celsius to 100.0 degrees Celsius?
what happens at 100.0 degrees Celsius?
what happens from 100.0 degrees Celsius to 140.0 degrees Celsius?
Then solve the full problem, showing work & units.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which traits do human embryos have that link them to a common ancestor with fish and reptiles? a. scales and tail b. gill slits and scales c. tail and gill slits d. hair and tail
Answers: 2

Chemistry, 22.06.2019 00:00
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1

Chemistry, 22.06.2019 00:00
Which type of bonding involves the complete transfer of a valence electron from a less electrogrative atom to a more electronegative one
Answers: 1

Chemistry, 22.06.2019 01:00
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3
You know the right answer?
A 200 g sample of water at 60.0 degrees Celsius is heated to water vapor at 140.0 degrees Celsius. E...
Questions






Mathematics, 05.09.2019 05:30

History, 05.09.2019 05:30


Mathematics, 05.09.2019 05:30

History, 05.09.2019 05:30

History, 05.09.2019 05:30



Chemistry, 05.09.2019 05:30

English, 05.09.2019 05:30

Mathematics, 05.09.2019 05:30

Mathematics, 05.09.2019 05:30

Arts, 05.09.2019 05:30




