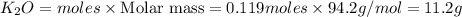Chemistry, 01.12.2020 07:30 ijustneedhelp29
PLEASE HELP
If 9.30 g of potassium reacts with 2.50 g of O2 to form K2O , what is the limiting reagent and what is the theoretical yield of the reaction?
Hint: write the balanced reaction
K - 39.10 g/mol
O - 15.999 g/mol
ANSWER CHOICES:
A.) O2 is limiting, 11.2 g of K2O formed
B.) K is limiting, 14.7 g of K2O formed
C.) K is limiting, 11.2 g of K2O formed
D.) O2 is limiting, 14.7 g of K2O formed
E.) O2 is limiting, 19.2 g of K2O formed

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2

Chemistry, 22.06.2019 17:30
What will most likely happen in the absence of a cell membrane? a) photosynthesis will not take place. b) the cell will not store food, water, nutrients, and waste. c) energy will not be released during cellular respiration. d) substances will pass in and out of the cell in an uncontrolled manner.
Answers: 1
You know the right answer?
PLEASE HELP
If 9.30 g of potassium reacts with 2.50 g of O2 to form K2O , what is the limiting reag...
Questions


Mathematics, 23.06.2021 16:00


Mathematics, 23.06.2021 16:00

Mathematics, 23.06.2021 16:00

World Languages, 23.06.2021 16:00

Computers and Technology, 23.06.2021 16:00

Mathematics, 23.06.2021 16:00


Mathematics, 23.06.2021 16:00

Mathematics, 23.06.2021 16:00

Mathematics, 23.06.2021 16:00


English, 23.06.2021 16:00


Mathematics, 23.06.2021 16:00




Social Studies, 23.06.2021 16:00

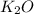 formed
formed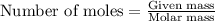
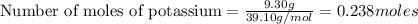
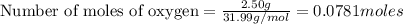
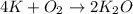
 require 1 mole of
require 1 mole of 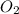
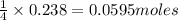 of
of 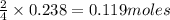 of
of