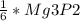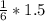Chemistry, 30.11.2020 18:20 descampbell2001
2. 1.5 moles of AgNO3 reacts with 0.5 mole of Mg3P2. Calculate the moles of excess reactant that remains at the end of the reaction. Include math to justify your answer.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1


Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2
You know the right answer?
2. 1.5 moles of AgNO3 reacts with 0.5 mole of Mg3P2. Calculate the moles of excess
reactant that re...
Questions






Physics, 08.10.2019 05:30



English, 08.10.2019 05:30

Mathematics, 08.10.2019 05:30

Chemistry, 08.10.2019 05:30

Biology, 08.10.2019 05:30

Mathematics, 08.10.2019 05:30



Biology, 08.10.2019 05:30


English, 08.10.2019 05:30

Mathematics, 08.10.2019 05:30