
Chemistry, 29.11.2020 14:40 johndoesnutz4690
Calculate the pH of a solution that is 0.291 M acetic acid and 0.123 M sodium acetate. The Ka of acetic acid is 1.76×10^–5 at 25°C. What is the pH of this mixture at 0°C?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:50
Where are chemicals found at work? a. only in cleaning products b. only in carpets and paint c. in every area of work d. only in food preparation submit
Answers: 1

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 19:50
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3

Chemistry, 23.06.2019 03:30
27 drag each label to the correct location on the image. a particular exosolar system has five planets in total: a, b, c, d, and e. the table lists the orbital periods of these planets in days. planet orbital period (days) a 600 b 80 c 1,000 d 500 e 100 move each planet to its orbit in the system.
Answers: 3
You know the right answer?
Calculate the pH of a solution that is 0.291 M acetic acid and 0.123 M sodium acetate. The Ka of ace...
Questions

History, 19.06.2021 07:00




Advanced Placement (AP), 19.06.2021 07:00

Physics, 19.06.2021 07:00







Spanish, 19.06.2021 07:10







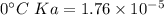
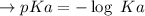
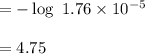
![pH = pKa + \log \frac{[sodium \ acetate]}{[acetic \ acid]}](/tpl/images/0932/3481/1f523.png)
![= 4.75 + \log \frac{[0.123]}{[0.291]}\\\\= 4.75+ \lg(0.422680412)\\\\=4.75-0.373987878\\\\=4.37601212\\\\=4.37](/tpl/images/0932/3481/5061d.png)



