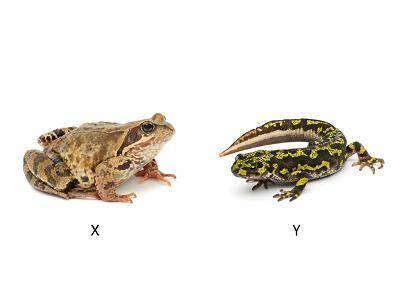
Study the diagram of the two animals.
Which statement best describes the animals?
Anima...

Chemistry, 25.11.2020 21:10 jordanmazer17
Study the diagram of the two animals.
Which statement best describes the animals?
Animal X begins life as a tadpole, and animal Y begins life as a larva that looks like the adult.
Animal X begins life as a larva that looks like the adult, and animal Y begins life as a tadpole.
Both animal X and animal Y begin life as a tadpole and change form as they develop.
Both animal X and animal Y begin life as a larva that looks like the adult.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 13:00
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1
You know the right answer?
Questions

Mathematics, 13.07.2019 08:00





History, 13.07.2019 08:00


History, 13.07.2019 08:00







Mathematics, 13.07.2019 08:00

History, 13.07.2019 08:00

Biology, 13.07.2019 08:00

Mathematics, 13.07.2019 08:00




