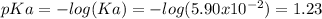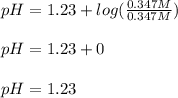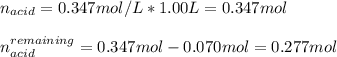Chemistry, 24.11.2020 21:00 carliehanson9908
A buffer solution is made that is 0.347 M in H2C2O4 and 0.347 M KHC2O4.
1. If Ka for H2C2O4 is 5.90E^-2, what is the pH of the buffer solution?
b. Write the net ionic equation for the reaction that occurs when 0.070 mol KOH is added to 1.00 L of the buffer solution.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Layers of rock containing fossils, like the layers illustrated here, are most likely composed of rocks.
Answers: 2

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 06:00
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

You know the right answer?
A buffer solution is made that is 0.347 M in H2C2O4 and 0.347 M KHC2O4.
1. If Ka for H2C2O4 is 5.90...
Questions

History, 10.02.2021 02:30

Mathematics, 10.02.2021 02:30


English, 10.02.2021 02:30

English, 10.02.2021 02:30

English, 10.02.2021 02:30

History, 10.02.2021 02:30



Chemistry, 10.02.2021 02:30


Mathematics, 10.02.2021 02:30


English, 10.02.2021 02:30


Biology, 10.02.2021 02:30

Mathematics, 10.02.2021 02:30

English, 10.02.2021 02:30


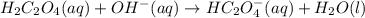
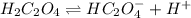
![pH=pKa+log(\frac{[base]}{[acid]} )](/tpl/images/0926/6955/33848.png)