

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 16:00
How do dying stars contribute to the formation of planets
Answers: 1

Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1
You know the right answer?
. Laughing gas is used by dentists as an anesthetic. If a 10.0 L tank of laughing gas contains 1.26...
Questions

Mathematics, 03.10.2019 06:30


Health, 03.10.2019 06:30

Chemistry, 03.10.2019 06:30


Mathematics, 03.10.2019 06:30

English, 03.10.2019 06:30

English, 03.10.2019 06:30


Mathematics, 03.10.2019 06:30

Spanish, 03.10.2019 06:30

Mathematics, 03.10.2019 06:30

History, 03.10.2019 06:30

Mathematics, 03.10.2019 06:30

English, 03.10.2019 06:30

Computers and Technology, 03.10.2019 06:30

Mathematics, 03.10.2019 06:30


Health, 03.10.2019 06:30

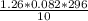 = 3.058atm
= 3.058atm

