
Chemistry, 23.11.2020 01:20 HarleyQuinn117
Using the balanced reaction below, drag and drop the terms into the correct location to solve the following problem:
If 1.5 moles of ethanol (C2H5OH) react, how many moles of water will be formed?
2 C2H5OH + 7 O2 --> 4 CO2 + 6 H2O
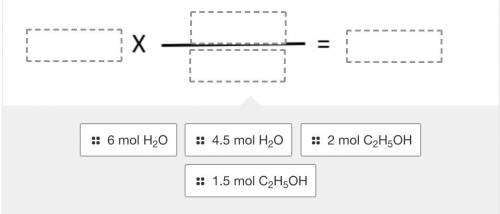

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 13:30
Which is the most likely way an automotive engineer would use chemistry
Answers: 1

Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1
You know the right answer?
Using the balanced reaction below, drag and drop the terms into the correct location to solve the fo...
Questions

English, 08.12.2020 22:50

History, 08.12.2020 22:50

Chemistry, 08.12.2020 22:50





Business, 08.12.2020 22:50

Mathematics, 08.12.2020 22:50

Mathematics, 08.12.2020 22:50

Mathematics, 08.12.2020 22:50




Mathematics, 08.12.2020 22:50

Biology, 08.12.2020 22:50


History, 08.12.2020 22:50

Mathematics, 08.12.2020 22:50




