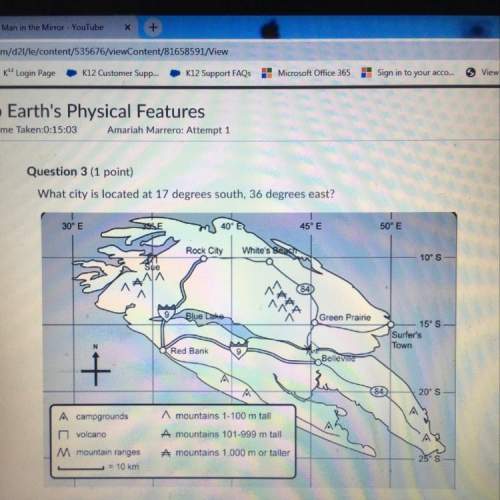
How does rusting differ from a combustion reaction?
O A. Rusting involves combining with oxygen, and combustion does not.
O B. Although both involve combining with oxygen, combustion is more rapid. O C. Combustion involves combining with oxygen, and rusting does not. O D. Rusting is a redox reaction, and combustion is not.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Pbco3 –> pbo+ co2. how many liters of carbon dioxide gas is produced from the decomposition of 32 grams of lead (ll) carbonate?
Answers: 1

Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3
You know the right answer?
How does rusting differ from a combustion reaction?
O A. Rusting involves combining with oxygen, an...
Questions

Business, 05.03.2022 14:00

Social Studies, 05.03.2022 14:00






Mathematics, 05.03.2022 14:00

Mathematics, 05.03.2022 14:00


Social Studies, 05.03.2022 14:00








Mathematics, 05.03.2022 14:00

Mathematics, 05.03.2022 14:00