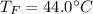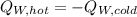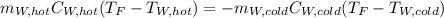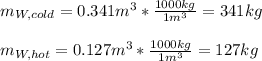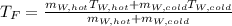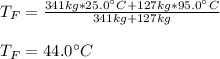Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 21:30
What is the correct name for the compound cocl3? a) cobalt(i) chloride b) cobalt(i) chlorate c) cobalt(ii) chlorate d) cobalt(iii) chloride
Answers: 1

Chemistry, 23.06.2019 07:40
)in the deacon process for the manufacture of chlorine, hcl and o2 react to form cl2 and h2o. sufficient air (21 mole% o2, 79% n2) is fed to provide 35% excess oxygen, and the fractional conversion of hcl is 85%. calculate the mole fractions of the product stream components.
Answers: 1

Chemistry, 23.06.2019 10:00
1.9 mol hcl and 3.9 mol naoh react according to the equation hcl + naoh −→ nacl + h2o . if the limiting reactant is hcl, calculate the amount of nacl formed.
Answers: 1

Chemistry, 23.06.2019 11:20
The chemical composition of soil varies with depth. an article in communications in soil science and plant analysis describes chemical analyses of soil taken from a farm in western australia. fifty specimens were each taken at depths 50 and 250 cm. at a depth of 50 cm, the average no3 concentration (in mg/l) was 88.5 with a standard deviation of 49.4. at a depth of 250 cm, the average concentration was 110.6 with a standard deviation of 51.5. find a 95% confidence interval for the difference in no3 concentrations at the two depths.
Answers: 1
You know the right answer?
If you have 0.341 m3 of water at 25.0 ∘C in an insulated container and add 0.127 m3 of water at 95.0...
Questions

Mathematics, 03.02.2021 03:50

Law, 03.02.2021 03:50



Social Studies, 03.02.2021 03:50


Mathematics, 03.02.2021 03:50

Social Studies, 03.02.2021 03:50

Mathematics, 03.02.2021 03:50

Mathematics, 03.02.2021 03:50


English, 03.02.2021 03:50




History, 03.02.2021 03:50

History, 03.02.2021 03:50


Mathematics, 03.02.2021 03:50