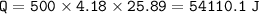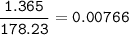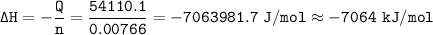When 1.365 g of anthracene, C14H10, is combusted in a bomb calorimeter that has a water jacket containing 500.0 g of water, the temperature of the water increases by 25.89°C. Assuming that the specific heat of water is 4.18 J/(g ∙°C), and that the heat absorption by the calorimeter is negligible, estimate the enthalpy of combustion per mole of anthracene.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 09:30
What are scientists who study fossils called? ( a ) astronomers. ( b ) biologists. ( c ) geologists. ( d ) paleontologists.
Answers: 2

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1
You know the right answer?
When 1.365 g of anthracene, C14H10, is combusted in a bomb calorimeter that has a water jacket conta...
Questions


Mathematics, 31.08.2019 00:30

Mathematics, 31.08.2019 00:30





Mathematics, 31.08.2019 00:30

Social Studies, 31.08.2019 00:30



Biology, 31.08.2019 00:30







Mathematics, 31.08.2019 00:30

Biology, 31.08.2019 00:30