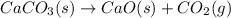Chemistry, 18.11.2020 17:10 julianbeaver76
Solid calcium carbonate decomposes to produce solid calcium oxide and carbon dioxide gas. Express your answer as a chemical equation. Identify all of the phases in your answer. CaCO,(s)-CaO(s) + CO2(g)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 17:10
In which block of the periodic table is uranium (u) found? s blockd blockp blockf block
Answers: 1

Chemistry, 23.06.2019 01:30
The solubility of barium nitrate is 9.02 g/100 g h2o at 20°c. a 15.2 g sample of barium nitrate is added to 200.0 g of water at 20°c. is the solution saturated, unsaturated, or supersaturated? a. unsaturated b. saturated c. supersaturated
Answers: 1

Chemistry, 23.06.2019 09:30
What lessons does the history and study of the periodic table offer to other fields of science, and the pursuit science more generally
Answers: 3
You know the right answer?
Solid calcium carbonate decomposes to produce solid calcium oxide and carbon dioxide gas. Express yo...
Questions

Computers and Technology, 26.06.2020 16:01




Mathematics, 26.06.2020 16:01

Computers and Technology, 26.06.2020 16:01


Mathematics, 26.06.2020 16:01

Advanced Placement (AP), 26.06.2020 16:01

Mathematics, 26.06.2020 16:01

Chemistry, 26.06.2020 16:01

History, 26.06.2020 16:01

Mathematics, 26.06.2020 16:01

Mathematics, 26.06.2020 16:01



English, 26.06.2020 16:01