

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
The wave shown on the electromagnetic spectrum disturb the medium it passes through a)different frequency. b)the same frequency .
Answers: 2

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1

Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2

Chemistry, 22.06.2019 19:30
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1
You know the right answer?
For the equilibrium that exists in an aqueous solution of nitrous acid (HNO2, a weak acid), the equi...
Questions




History, 09.04.2020 20:28

Spanish, 09.04.2020 20:28










Mathematics, 09.04.2020 20:28


Physics, 09.04.2020 20:28

English, 09.04.2020 20:28

Chemistry, 09.04.2020 20:28

History, 09.04.2020 20:28

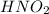
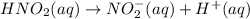
![k_a = \frac{[NO_{2}^{-}][H^{+}]}{HNO_{2}}](/tpl/images/0908/7640/67909.png)


