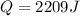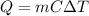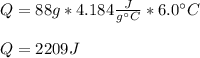Chemistry, 16.11.2020 22:30 miahbaby2003p2af1b
A sample of water (88 grams) had a temperature change of 6.0 ℃. What was the heat change of this sample? (q = m c ΔT, c = 4.18 J/ g ℃)

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:00
Look at the reaction below: ca(hco3)2 --> caco3 + co2 + h2o first, balance the reaction. once balanced, use dimensional analysis or another method to find out how many moles of carbon dioxide will be produced if we start with 16.5 moles of calcium bicarbonate (calcium hydrogen carbonate). = mol of co2 number needs to be reported to three significant figures.
Answers: 1

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1


Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1
You know the right answer?
A sample of water (88 grams) had a temperature change of 6.0 ℃. What was the heat change of this sam...
Questions


History, 08.02.2021 19:20

History, 08.02.2021 19:20

English, 08.02.2021 19:20






Mathematics, 08.02.2021 19:20



Physics, 08.02.2021 19:20

Mathematics, 08.02.2021 19:20


Mathematics, 08.02.2021 19:20