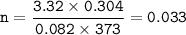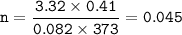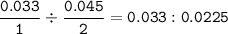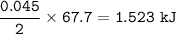CHEMWORK The preparation of NO2(g) from N2(g) and O2(g) is an endothermic reaction: N2(g) + O2(g) + NO2(g) (unbalanced) The enthalpy change of reaction for the balanced equation (with lowest whole-number coefficients) is AH = 67.7 kJ. If 304 ml N2 (9) at 100ºC and 3.32 atm and 410 ml 0,(9) at 100°C and 3.32 atm are mixed, what amount of heat is necessary to synthesize the maximum yield of NO2(g) ?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2
You know the right answer?
CHEMWORK The preparation of NO2(g) from N2(g) and O2(g) is an endothermic reaction: N2(g) + O2(g) +...
Questions


Physics, 04.12.2019 20:31


Social Studies, 04.12.2019 20:31

English, 04.12.2019 20:31


Mathematics, 04.12.2019 20:31

Mathematics, 04.12.2019 20:31


Mathematics, 04.12.2019 20:31


History, 04.12.2019 20:31


Mathematics, 04.12.2019 20:31