
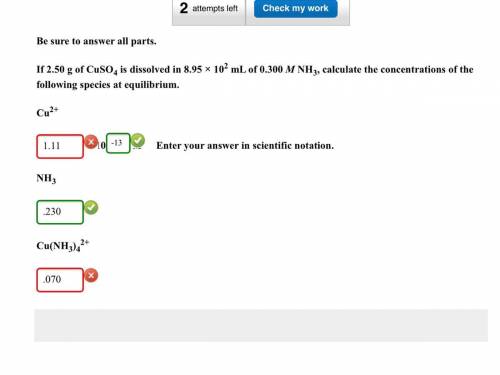
If 2.50g of CuSO4 is dissolved in 8.95 x 10^2 mL of 0.300 M NH3, calculate the concentrations of the following species at equilibrium: Cu^2+, NH3, Cu(NH3)4^2.
I tried to solve and came up with the following-
Cu^2+ = 1.1149x10^-13 (Which was all wrong except for the 10^-13)
NH3 = .230 (Which was correct)
Cu(NH3)4^2+ = 0.069720 (Which was wrong)
Can someone please show me where I am going wrong.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1


Chemistry, 23.06.2019 01:00
Which statement characterizes synthetic polymers? a. they come from animals and plants. b. they are found in nature. c. they are made in a lab. d. they are components of starch.
Answers: 1

Chemistry, 23.06.2019 08:00
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 2
You know the right answer?
If 2.50g of CuSO4 is dissolved in 8.95 x 10^2 mL of 0.300 M NH3, calculate the concentrations of the...
Questions


Biology, 06.07.2019 13:00


Mathematics, 06.07.2019 13:00

Physics, 06.07.2019 13:00

English, 06.07.2019 13:00


Mathematics, 06.07.2019 13:00



English, 06.07.2019 13:00

Mathematics, 06.07.2019 13:00





Mathematics, 06.07.2019 13:00

Mathematics, 06.07.2019 13:00




