
1. The diagram shows an electrochemical cell with copper (left) and zinc (right) strips.
(attached below)
response, do the following:
• Label all parts (1–9), including the solutions in each beaker and the connecting tube.
• Label which cell is the cathode and which cell is the anode. Include the charge on each strip.
• Show, or describe in detail, the flow of electrons.
• Describe what type of electrochemical cell is pictured. Explain how the cell works. Include the oxidation and reduction half-reactions in your explanation.
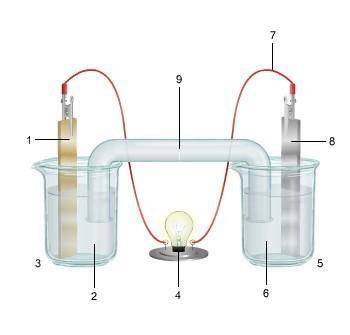

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2

Chemistry, 22.06.2019 12:10
|using the periodic tablewarm-upuse the periodic table in the tools bar to answer the following questions.what elemental classification does oxygen belongto? done
Answers: 3

Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3
You know the right answer?
1. The diagram shows an electrochemical cell with copper (left) and zinc (right) strips.
(attached...
Questions


History, 20.10.2020 18:01

Mathematics, 20.10.2020 18:01


Mathematics, 20.10.2020 18:01



Mathematics, 20.10.2020 18:01

History, 20.10.2020 18:01

Social Studies, 20.10.2020 18:01

Mathematics, 20.10.2020 18:01




History, 20.10.2020 18:01

Mathematics, 20.10.2020 18:01

English, 20.10.2020 18:01


World Languages, 20.10.2020 18:01

Mathematics, 20.10.2020 18:01




