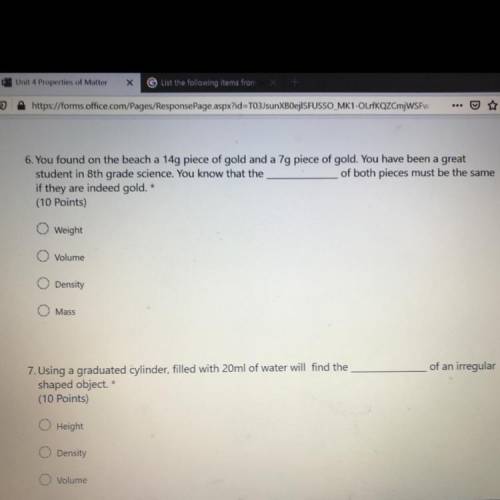
:answer question number 6
...

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 04:20
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1

Chemistry, 23.06.2019 08:00
Suppose a pair of chemical compounds a and b can react in two different ways: a + b -> c reaction 1 gives product c. a + b -> d reaction 2 gives product d. the following facts are known about the two reactions: . reaction 1 is endothermic and reaction 2 is exothermic. if a reaction vessel is charged (filled) with a and b , then at first d is produced faster than c. use these facts to sketch a qualitative reaction energy diagram for both reactions. note: because these sketches are only qualitative, the energies don? t have to be exact. they only have to have the right relationship to each other. for example, if one energy is less than another, that fact should be clear in your sketch.
Answers: 3

Chemistry, 23.06.2019 08:20
At which temperature would a reaction with ah= -220 kj/mol and as=-0.05 kj/(mol-k) be spontaneous?
Answers: 2

Chemistry, 23.06.2019 14:30
The hammering on a train track is often heard twice by workers farther down the track; first as the sound travels through the steel and second as the sound travels through the air. this suggests which graph is true?
Answers: 1
You know the right answer?
Questions

Social Studies, 20.11.2020 02:50

Arts, 20.11.2020 02:50

Mathematics, 20.11.2020 02:50


Chemistry, 20.11.2020 02:50

Mathematics, 20.11.2020 02:50

Social Studies, 20.11.2020 02:50


Mathematics, 20.11.2020 02:50

Advanced Placement (AP), 20.11.2020 02:50






Biology, 20.11.2020 02:50


Health, 20.11.2020 02:50