
For many purposes we can treat butane (C4H10) as an ideal gas at temperatures above its boiling point of - 1. °C.
Suppose the pressure on a 3.0 m3 sample of butane gas at 19.0°C is reduced to one-third its initial value.
Is it possible to change the temperature of the butane at the same time such that
the volume of the gas doesn't change?
If you answered yes, calculate the new temperature of the gas. Round your
answer to the nearest °C.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:20
Much of the general structure and physical properties of the interior of the earth are inferred from: a)deep oil and gas bore holes b)geologic investigations c)analysis of seismic waves d) study of volcanoes
Answers: 1

Chemistry, 22.06.2019 05:30
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 19:20
For a research project, a student decided to test the effect of the lead(ii) ion (pb2+) on the ability of salmon eggs to hatch. this ion was obtainable from the water‐soluble salt, lead(ii) nitrate, which the student decided to make by the following reaction. pbo(s) + 2 hno3(aq) → pb(no3)2(aq) + h2o losses of product for various reasons were expected, and a yield of 86.0% was expected. in order to have 5.00 g of product at this yield, how many grams of pbo should be reacted? (assume that sufficient nitric acid, hno3, would be used.)
Answers: 1
You know the right answer?
For many purposes we can treat butane (C4H10) as an ideal gas at temperatures above its boiling poin...
Questions

Mathematics, 19.03.2021 21:00

Mathematics, 19.03.2021 21:00




Mathematics, 19.03.2021 21:00

Biology, 19.03.2021 21:00



Computers and Technology, 19.03.2021 21:00

Mathematics, 19.03.2021 21:00

English, 19.03.2021 21:00





English, 19.03.2021 21:00

Biology, 19.03.2021 21:00


History, 19.03.2021 21:00

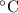 .
.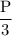
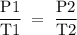
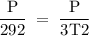
 3T2 = P
3T2 = P 

