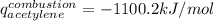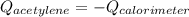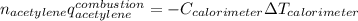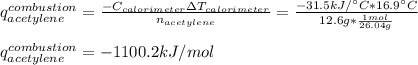Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 23.06.2019 00:00
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1

Chemistry, 23.06.2019 01:00
Which polymers are most closely related? a. protein and nucleic acids b. cellulose and starch c. nucleic acids and starch d. nucleic acids and cellulose
Answers: 2

Chemistry, 23.06.2019 07:00
The following transition occurs at a molecular level for a substance. what transition corresponds to this change in microscopic structure? the carbon dioxide molecules on the left are in a regular, tightly packed pattern. after heating, it becomes much lower density. a. melting b. boiling c. sublimation d. freezing
Answers: 1
You know the right answer?
The heat capacity of a bomb calorimeter was determined to be 31.5 kJ/oC. A 12.6 g sample of acetylen...
Questions


English, 03.02.2021 23:40

Computers and Technology, 03.02.2021 23:40




Mathematics, 03.02.2021 23:40

Biology, 03.02.2021 23:40


History, 03.02.2021 23:40

Mathematics, 03.02.2021 23:40

Mathematics, 03.02.2021 23:40

Health, 03.02.2021 23:40

Mathematics, 03.02.2021 23:40





Social Studies, 03.02.2021 23:40

Mathematics, 03.02.2021 23:40