
Chemistry, 28.10.2020 17:00 ummmmmmmmmmmm
An iron ore sample contains plus other impurities. A 896-g sample of impure iron ore is heated with excess carbon, producing 182 g of pure iron by the following reaction: What is the mass percent of FeO2 in the impure iron ore sample

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:30
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1

Chemistry, 23.06.2019 01:00
If a straight-chain hydrocarbon is a gas at room temperature, how many carbon atoms will it have? a. 6 carbon atoms b. 12 carbon atoms c. 24 carbon atoms d. 3 carbon atoms
Answers: 1
You know the right answer?
An iron ore sample contains plus other impurities. A 896-g sample of impure iron ore is heated with...
Questions


Mathematics, 13.09.2019 22:10


History, 13.09.2019 22:10

Mathematics, 13.09.2019 22:10


History, 13.09.2019 22:10




Mathematics, 13.09.2019 22:10


Chemistry, 13.09.2019 22:10

Spanish, 13.09.2019 22:10

Mathematics, 13.09.2019 22:10





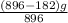 × 100
× 100 

