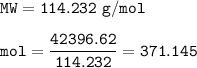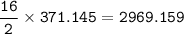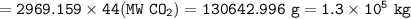Answers: 2
Another question on Chemistry

Chemistry, 20.06.2019 18:02
All living organisms are composed of a. at least three cells. b. one or more cells. c. only one cell. d. at least 100 cells.
Answers: 2

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1
You know the right answer?
What mass of CO2 (in kilograms) does the combustion of a 16-gallon tank of gasoline release into the...
Questions


Mathematics, 15.07.2019 00:40





English, 15.07.2019 00:40

History, 15.07.2019 00:40


Advanced Placement (AP), 15.07.2019 00:40




Computers and Technology, 15.07.2019 00:50


Social Studies, 15.07.2019 00:50


Biology, 15.07.2019 00:50

History, 15.07.2019 00:50