
Chemistry, 26.10.2020 17:20 justinchou814
Calculate the mass defect for 239U239U, which has a mass of 239.05429 amuamu . (The mass of 11H11H is 1.00783 amuamu, and the mass of a neutron is 1.00866 amuamu .)

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3


Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2
You know the right answer?
Calculate the mass defect for 239U239U, which has a mass of 239.05429 amuamu . (The mass of 11H11H i...
Questions


English, 08.02.2021 20:50

History, 08.02.2021 20:50




Computers and Technology, 08.02.2021 20:50



History, 08.02.2021 20:50

Physics, 08.02.2021 20:50

Mathematics, 08.02.2021 20:50




Business, 08.02.2021 20:50

Chemistry, 08.02.2021 20:50

Spanish, 08.02.2021 20:50

Computers and Technology, 08.02.2021 20:50

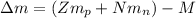
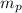 : is the proton mass = 1.00783 amu
: is the proton mass = 1.00783 amu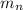 : is the neutron mass = 1.00866 amu
: is the neutron mass = 1.00866 amu ![\Delta m = (Zm_{p} + Nm_{n}) - M = [92*1.00783 amu + (239 - 92)*1.00866 amu] - 239.05429 amu = 1.93909 amu](/tpl/images/0840/4283/ccb79.png)


