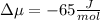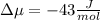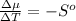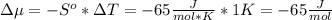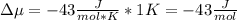Chemistry, 26.10.2020 16:40 corey36dylon
The standard molar entropy of liquid water at 273.15 K is 65 J K−1 mol−1, and that of ice at the same temperature is 43 J K−1 mol−1. Calculate the change in chemical potential of liquid water and of ice when the temperature is increased by 1 K from the normal melting point. Giving your reasons, explain which phase is thermodynamically the more stable at the new temperature.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 21.06.2019 23:10
Nitrogen (n), phosphorus (p), and potassium (k) are the main nutrients in plant fertilizers. according to an industry convention, the numbers on the label refer to the mass percents of n, p2o5, and k2o, in that order. calculate the n: p: k ratio of a 30: 10: 10 fertilizer in terms of moles of each element, and express it as x: y: 1.0.
Answers: 1

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1
You know the right answer?
The standard molar entropy of liquid water at 273.15 K is 65 J K−1 mol−1, and that of ice at the sam...
Questions


History, 18.10.2019 15:30

Mathematics, 18.10.2019 15:30



Chemistry, 18.10.2019 15:30

History, 18.10.2019 15:30

Mathematics, 18.10.2019 15:30





English, 18.10.2019 15:30

History, 18.10.2019 15:30

English, 18.10.2019 15:30


History, 18.10.2019 15:50

English, 18.10.2019 15:50

History, 18.10.2019 15:50