
Chemistry, 22.10.2020 21:01 evanwall91
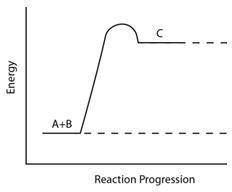
Consider the reaction pathway graph below.
Which statement accurately describes this graph?
A) It represents an endothermic reaction because the product has more energy than the reactants.
B) It represents an exothermic reaction because the product has more energy than the reactants.
C) It represents an endothermic reaction because the reactants have more energy than the product.
D) It represents an exothermic reaction because the reactants have more energy than the product.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1


Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3
You know the right answer?
Consider the reaction pathway graph below.
Which statement accurately describes this graph?
A...
A...
Questions

Mathematics, 20.11.2020 18:20


Mathematics, 20.11.2020 18:20


Mathematics, 20.11.2020 18:20



Biology, 20.11.2020 18:20

Mathematics, 20.11.2020 18:20

Mathematics, 20.11.2020 18:20




Health, 20.11.2020 18:20

Biology, 20.11.2020 18:20

Mathematics, 20.11.2020 18:20


Health, 20.11.2020 18:20


Business, 20.11.2020 18:20



