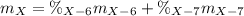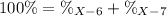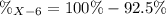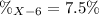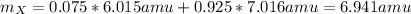Chemistry, 22.10.2020 20:01 BreBreDoeCCx
Element X has two natural isotopes: X-6 (6.015 amu) and X-7 (7.016 amu). Calculate the atomic mass of element X given the abundance of X-7 is 92.5%

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1
You know the right answer?
Element X has two natural isotopes: X-6 (6.015 amu) and X-7 (7.016 amu). Calculate the atomic mass o...
Questions




Biology, 17.09.2019 10:10



Physics, 17.09.2019 10:10

Social Studies, 17.09.2019 10:10

Computers and Technology, 17.09.2019 10:10

History, 17.09.2019 10:10


English, 17.09.2019 10:10


History, 17.09.2019 10:10

Health, 17.09.2019 10:10


Chemistry, 17.09.2019 10:10

Biology, 17.09.2019 10:10

Physics, 17.09.2019 10:10

History, 17.09.2019 10:10