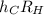Chemistry, 21.10.2020 04:01 logsdonella
With some manipulation, the Rydberg equation can be rewritten in the form
E=constant×(1nf2−1ni2)
which allows you to calculate the energy of the emitted light. Express this constant in terms of the constants h, c, and RH using relationships between wavelength and energy as well as the Rydberg equation from the introduction.
Express the constant in terms of h and c, and RH.
Please help me with this question?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Write the symbol for every chemical element that has atomic number greater than 3 and atomic mass less than 12.0 u.
Answers: 1

Chemistry, 22.06.2019 08:30
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1

Chemistry, 22.06.2019 13:00
Lab reagent, hypothesis test.a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl.these six measurements are assumed to be an srs of all possible measurements from solution.they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution.carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1

Chemistry, 23.06.2019 00:00
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3
You know the right answer?
With some manipulation, the Rydberg equation can be rewritten in the form
E=constant×(1nf2−1ni2)
Questions



Mathematics, 26.02.2021 19:40




Chemistry, 26.02.2021 19:40

History, 26.02.2021 19:40

Biology, 26.02.2021 19:40



Mathematics, 26.02.2021 19:40

Physics, 26.02.2021 19:40


English, 26.02.2021 19:40

English, 26.02.2021 19:40

Medicine, 26.02.2021 19:40

Mathematics, 26.02.2021 19:40