
Chemistry, 17.10.2020 20:01 emmaschloegl21x
A 57.07 g sample of a substance is initially at 24.3°C. After absorbing of 2911 J of heat, the temperature of the substance is 116.9 CWhat is the specific heat (SH) of the substance?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 23.06.2019 03:00
Determine type of reaction & predict the product c3h12+o2 =
Answers: 1


Chemistry, 23.06.2019 13:30
How does water evaporating from a glass show that matter is made up of particles? a. the heat energy from the air causes the glass to fill up with water particles. b. the liquid water particles turn into water vapor that spreads in the air. c. the particles of the glass dissolve in water and cause it to evaporate. d. the tiny particles of the glass evaporate and seem to disappear.
Answers: 2
You know the right answer?
A 57.07 g sample of a substance is initially at 24.3°C. After absorbing of 2911 J of heat, the tempe...
Questions

Business, 19.01.2022 05:40




Mathematics, 19.01.2022 05:40


SAT, 19.01.2022 05:40



Mathematics, 19.01.2022 05:40


Computers and Technology, 19.01.2022 05:40

Social Studies, 19.01.2022 05:40

Mathematics, 19.01.2022 05:40

Biology, 19.01.2022 05:40

Mathematics, 19.01.2022 05:40

English, 19.01.2022 05:40

SAT, 19.01.2022 05:40

Mathematics, 19.01.2022 05:50

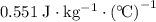 .
.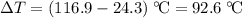 .
.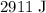 of energy to raise the temperature of this sample by
of energy to raise the temperature of this sample by 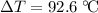 . Therefore, raising the temperature of this sample by
. Therefore, raising the temperature of this sample by 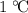 (unit temperature) would take only
(unit temperature) would take only 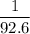 as much energy. That corresponds to approximately
as much energy. That corresponds to approximately 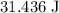 of energy.
of energy.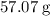 of this material by
of this material by 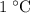 . Therefore, it would take only
. Therefore, it would take only 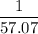 as much energy to raise the temperature of
as much energy to raise the temperature of 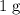 (unit mass) of this material by
(unit mass) of this material by 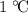 . That corresponds to approximately
. That corresponds to approximately 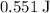 of energy.
of energy.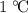 . Therefore, by definition, the specific heat of this material would be approximately
. Therefore, by definition, the specific heat of this material would be approximately 

