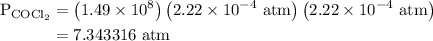Chemistry, 29.09.2019 09:00 charae0185
The kp for the reaction below is 1.49 × 108 at 100.0°c: co(g) + cl2(g) → cocl2(g) in an equilibrium mixture of the three gases, pco = pcl2 = 2.22 × 10-4 atm. the partial pressure of the product, phosgene (cocl2), is atm.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
If a plot weight (in g) vs. volume (in ml) for a metal gave the equation y= 13.41x and r^2=0.9981 what is the density of the metal?
Answers: 2

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 12:20
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l.s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3
You know the right answer?
The kp for the reaction below is 1.49 × 108 at 100.0°c: co(g) + cl2(g) → cocl2(g) in an equilibrium...
Questions

Mathematics, 13.02.2020 08:01

Biology, 13.02.2020 08:01

History, 13.02.2020 08:01

Mathematics, 13.02.2020 08:01

Mathematics, 13.02.2020 08:01

Mathematics, 13.02.2020 08:01



Mathematics, 13.02.2020 08:01

Mathematics, 13.02.2020 08:01

Biology, 13.02.2020 08:01

History, 13.02.2020 08:01


Mathematics, 13.02.2020 08:02

Health, 13.02.2020 08:02


Mathematics, 13.02.2020 08:02

Mathematics, 13.02.2020 08:02

Engineering, 13.02.2020 08:02

Mathematics, 13.02.2020 08:02

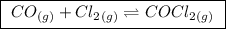 (a balanced reaction)
(a balanced reaction)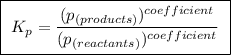
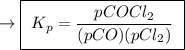
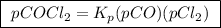
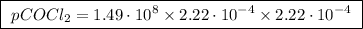
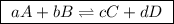 ,
,![\boxed{ \ K_c = \frac{[C]^c[D^d]}{[A]^a[B]^b} \ }](/tpl/images/0273/3667/d057b.png)
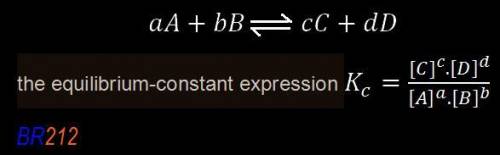
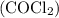 is
is 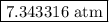 .
.
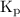 .
.
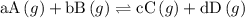
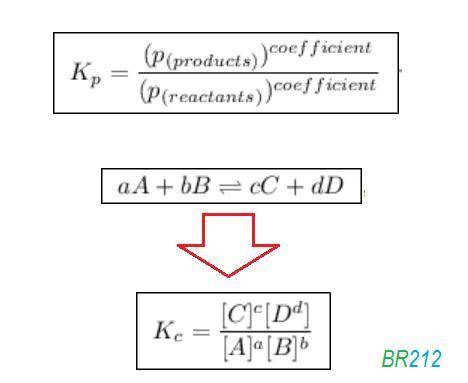
![{{\text{K}}_{\text{p}}} = \dfrac{{{{\left[ {{{\text{P}}_{\text{C}}}} \right]}^{\text{c}}}{{\left[ {{{\text{P}}_{\text{D}}}} \right]}^{\text{d}}}}}{{{{\left[ {{{\text{P}}_{\text{A}}}} \right]}^{\text{a}}}{{\left[ {{{\text{P}}_{\text{B}}}} \right]}^{\text{b}}}}}](/tpl/images/0273/3667/ee3bd.png)
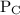 and
and 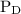 are partial pressures of C and D respectively.
are partial pressures of C and D respectively.
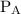 and
and 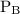 are partial pressures of A and B respectively.
are partial pressures of A and B respectively.
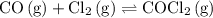
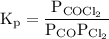 ...... (1)
...... (1)
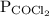 is partial pressure of
is partial pressure of 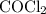 .
.
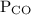 is partial pressure of CO.
is partial pressure of CO.
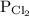 is partial pressure of
is partial pressure of 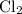 .
.
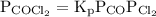 …… (2)
…… (2)
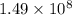 for
for 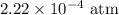 for
for